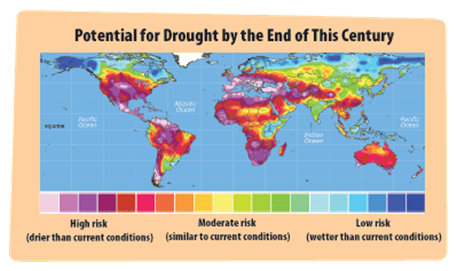
In 2015, the United Nations (UN, 193 countries) agreed to adopt the 2030 Agenda for upon laying out 17 Sustainable Development Goals (SDGs) which shall be completed by the year 2030. Our responsibility towards “Climate Change" and “Global Warming Mitigation" can be resolved upon implementation of carbon capture and sequestration (CCS), utilization (CCU), recycling (CCR), as well as direct air capture technologies. Earth has seen the increased devastating effects of global warming as in melting ice caps, increased sea levels, floods, storms, habitat loss and extinctions which will result in global droughts.

(Source: United Nations)
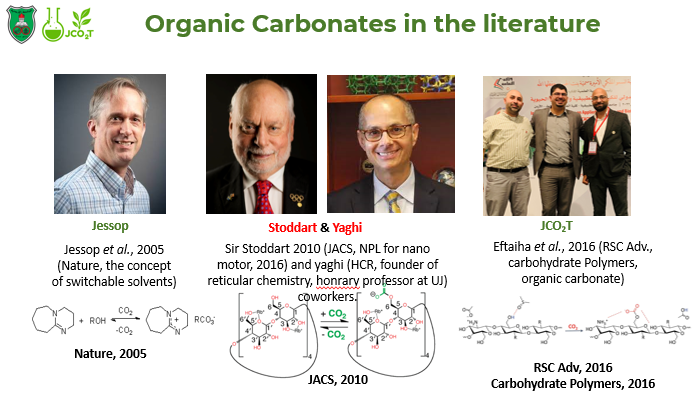
Our research group devoted its message towards the realization of climate action as a sustainable development goal (SDG 13) along with other SDGs (4,
9 and
17) as a parallel target to be achieved
via research-based teaching methodology. This can be rationalized by our dissemination and spreading worldwide awareness (reviews, research articles, and communications) within the local community (School projects, Conferences and Oral presentations).
(Source: EPA)
Our research
Research Interests: Catalysis, nanomaterials, CO2 fixation, Organic and Polymer Chemistry (condensation, radical, coordination), Green chemistry, CO2 Sequestration, surfactants (conventional vs. gemini), biomimicry (ongoing M.Sc. project), biomaterial organocatalysis/CO2 capture.
The production of nanomaterials, polymers, organic and inorganic materials for the capture of greenhouse gases (GHGs) such as carbon dioxide (CO2) and its sequestration throughout physical/chemical fixation using
green sorbents for CO2 capturing as a new addition in Green chemistry by our research group. Further, We do heavily implement both stoichiometric or catalytic (homogenous and heterogeneous) approaches for the production of fine chemicals as in
cyclic carbonates (CCs),
carbamates,
ionic organic carbonates,
urethanes, and
ureas. Further, organic substrates can be utilized from
biomaterials,
surfactants, and commercially available materials and/or their synthesis/implementation. In addition, from an academia point of view, proper catalytic cycles mapping and overall proven mechanisms are a must to comprehend the stability of the GHG gas which will decrease the energy bill for its industrialization purposes. Green chemistry is a must to be followed for the development of these materials. It deals with ecofriendly approaches for the manipulation of chemical reactions which is the first of its kind to be implemented in the Kingdom out of CO2 as a
C1-stock material.
The research group interests
A. Green Sorbents for CO2 capturing (Polymeric, nanomaterials, Organic molecules, biomaterials) for the mitigation of climate changes
The excessive use of different varieties of compounds with different tethering made us understand the mechanistic approach for their capture. We have made it possible to prepare different types of compounds/intermediates (ionic organic carbonates) which were a debate reported in the literature (carbamic versus carbamate). Several spectroscopic/spectrometric techniques are utilized for the analysis/detection of such intermediates.
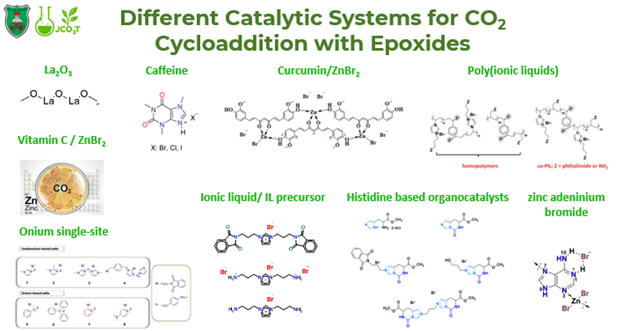
B. Synthesis of different Homogeneous/Heterogenous catalytic systems for CO2 fixation into fine chemicals (Organocatalysis, Polymeric materials, and inorganic complexes)
The production of different catalytic systems is highly crucial for the cost versus efficiency demand for the optimization and functionalization of these motifs with emphasis on using bio-based resources for the production of CCs as a result of the cycloaddition reaction of CO2 with epoxides
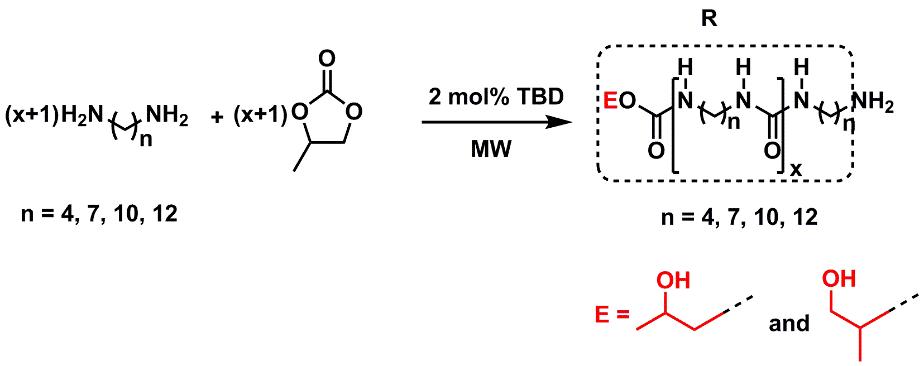
C. Synthesis of novel Materials applying Green Chemistry with focus on ecofriendly approaches for Urea/urethane formation
The importance of the production of these fine chemicals (ureas/urethanes) that can be considered a billion-dollar industry that can be obtained using non-phosgene, non-isocyanate routes using CO2 and/or CCs as carbonylating agents following organocatalyzed microwave/thermal based technologies. A new synthetic approach is followed for the first time in Jordan with concerned with biomimicry for CO2 fixation is under progress.

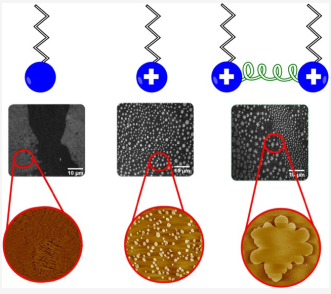
D. Synthesis of surfactants
Surfactants (surface active agents) are powerful target molecules that are considered as a powerful industry that are vastly used in different applications. Our aim is to synthesize these materials and study their physical/chemical behavior using micro/nano techniques solid/liquid interfaces.

The research group in Brief
The Jordanian CO2 Team (JCO2T) started its work officially at the University of Jordan in 2017 with its founder
Dr. Abdussalam K. Qaroush (Associate Professor of organic & Polymer Chemistry), although established in 2015 in the Hashemite University as an
alumni unionpartnership of
Dr. Alaa F. Eftaiha (Associate Professor of physical Chemistry) and managed to expand its partnership with
Dr. Khaleel I. Assaf ( Associate Professor of Organic Chemistry, Al-Balqa Applied University)and later joined by
Dr. Feda M. Alqaisi (Assistant Professor of inorganic Chemistry, the Hashemite University). The research group highlights its fields of interest towards green chemistry principles (GCPs) and its implementation in CO2 capture, utilization, and recycling (as a future goal). Along with Catalysis, polymer chemistry, ionic liquids, coordination and inorganic chemistry, nanotechnology, Green surfactants synthesis. The research group partners are the Technical University of Munich (TUM), Saskatchewan University, Helsinki University, and Constructor University (previously known as the university of Jacobs Bremen). Moreover, the research group managed to partner with
Canada Research Chair of Green chemistry, Professor Phillip Jessop working on Switchable polarity solvents CO2 as a Trigger for Switchable Systems, Water/Solute Separations, Biomass Conversion and Separation.