Breast cancer is the second-most prevalent cancer globally, closely following lung cancer. In Jordan, it is the most widespread cancer. The disease can be broadly classified into four types according to the expression of receptors: estrogen receptor-positive luminal A and B, human epithelial growth factor receptor 2 (HER2)-enriched, and triple-negative breast cancer (TNBC). Whereas the first three types can be treated by ER and HER2 antagonists, respectively, the latter does not have any therapeutic targets.
There is renewed interest in using the androgen receptor (AR) for the treatment of the disease, particularly AR-positive TNBC, especially since the androgenic hormone dihydrotestosterone (DHT), which acts through AR, plays a major role in the biology of breast cancer.
The research theme of our laboratory is to understand the biological role of DHT and AR on breast cancer, particularly TNBC, via the implementation of a plethora of cellular and molecular biology techniques and breast cancer cell lines and human tissue samples as model systems. There is also special interest in elucidating the role of non-coding RNAs in mediating the actions of AR. Our main findings suggest that:
- DHT induces partial epithelia-mesenchymal transition and cell migration via separate molecular mechanisms.
- DHT induces chemoresistance of breast cancer cells.
- MicroRNA molecules may mediate the actions of DHT.
Ongoing and future directions:
- Androgen-mediated regulation of non-coding RNA molecules (microRNA, circular RNA, and long noncoding RNA) and its biological implications.
- The molecular mechanisms and clinical significance of androgen-induced chemoresistance.


Relevant publications:
1- The Association of RGS2 and Slug in the Androgen-induced Acquisition of Mesenchymal Features of Breast MDA-MB-453 Cancer Cells.
Endocr Res. 2022 Feb-May;47(2):64-79. doi: 10.1080/07435800.2022.2036752. Epub 2022 Feb 16. Alsafadi DB, Abdullah MS, Bawadi R, Ahram M.
BACKGROUND:
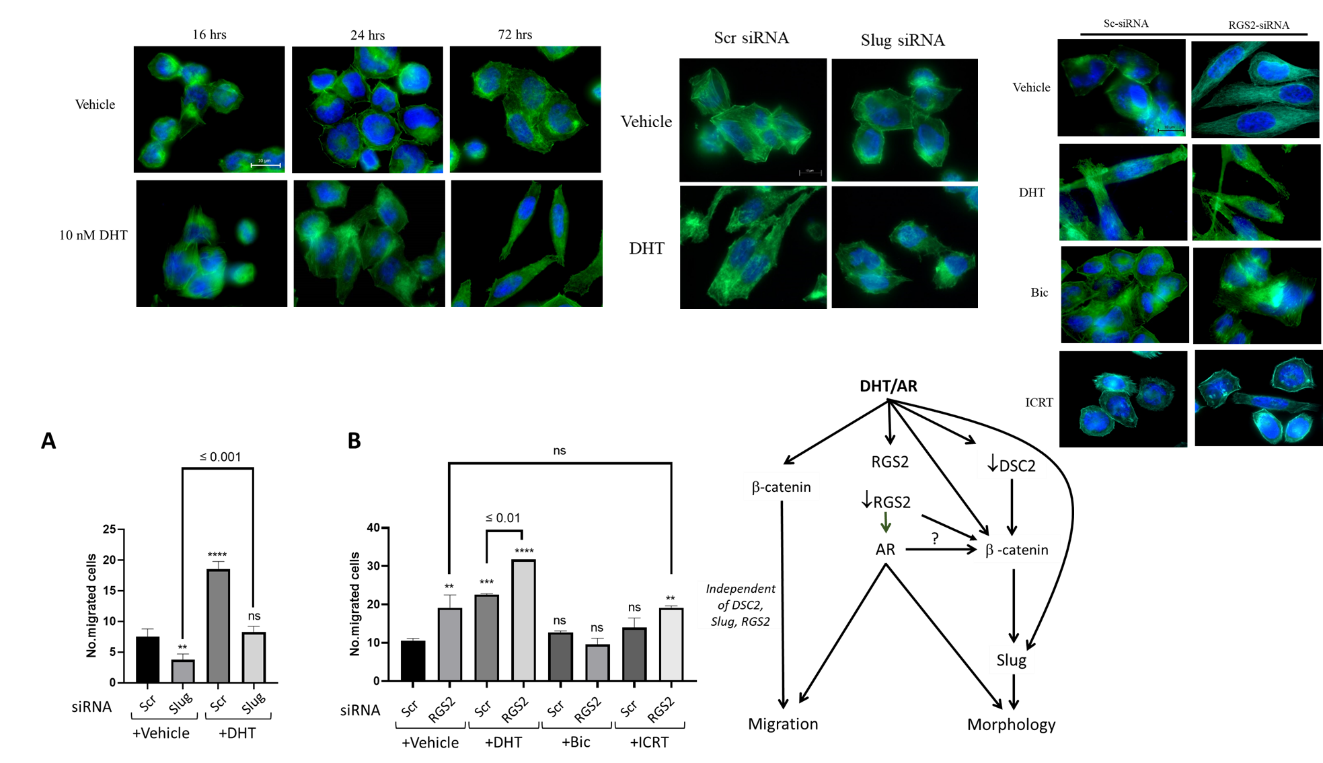
Epithelial-mesenchymal transition (EMT) of tumor cells is a prerequisite to cancer cell invasion and metastasis. This process involves a network of molecular alterations. Androgen receptor (AR) plays an important role in the biology of breast cancers, particularly those dependent on AR expression like luminal AR (LAR) breast cancer subtype. We have recently reported that the AR agonist, dihydrotestosterone (DHT), induces a mesenchymal transition of MDA-MB-453 cells, concomitant with transcriptional up-regulation of Slug and regulator of G protein signaling 2 (RGS2).
OBJECTIVE:
The role of Slug and RGS2 in mediating the DHT-induced effects in these cells was investigated.
METHODS:
MDA-MB-453 cells were used as a model system of LAR breast cancer. Immunofluorescence was used to examine cell morphology and protein localization. Protein expression was analyzed by immunoblotting. Protein localization was confirmed by cell fractionation followed by immunoblotting. Protein-protein interaction was confirmed by co-immunoprecipitation followed by immunoblotting. Transwell membranes were used to assess cell migration. Transfection of cells with siRNA molecules that target Slug and RGS2 mRNA was utilized to delineate the modes of action of these two molecules.
RESULTS:
Treatment of MDA-MB-453 cells with DHT induced the expression of both proteins. In addition, AR-Slug, AR-RGS2, and Slug-RGS2 interactions were observed shortly after AR activation. Knocking down Slug abrogated the basal, but not the DHT-induced, cell migration and blocked DHT-induced mesenchymal transition. On the other hand, RGS2 knocked-down cells had an increased level of Slug protein and assumed mesenchymal cell morphology with induced migration, and the addition of DHT further elongated cell morphology and stimulated their migration. Inhibition of AR or β-catenin reverted the RGS2 knocked-down cells to the epithelial phenotype, but only inhibition of AR blocked their DHT-induced migration.
CONCLUSIONS:
These results suggest the involvement of RGS2 and Slug in a complex molecular network regulating the DHT-induced mesenchymal features in MDA-MB-453 cells. The study may offer a better understanding of the biological role of AR in breast cancer toward devising AR-based therapeutic strategies.
2- Androgen downregulates desmocollin-2 in association with induction of mesenchymal transition of breast MDA-MB-453 cancer cells.
Cytoskeleton (Hoboken). 2021 Aug;78(8):391-399. doi: 10.1002/cm.21691. Epub 2022 Jan 24. Ahram M, Abdullah MS, Mustafa SA, Alsafadi DB, Battah AH.
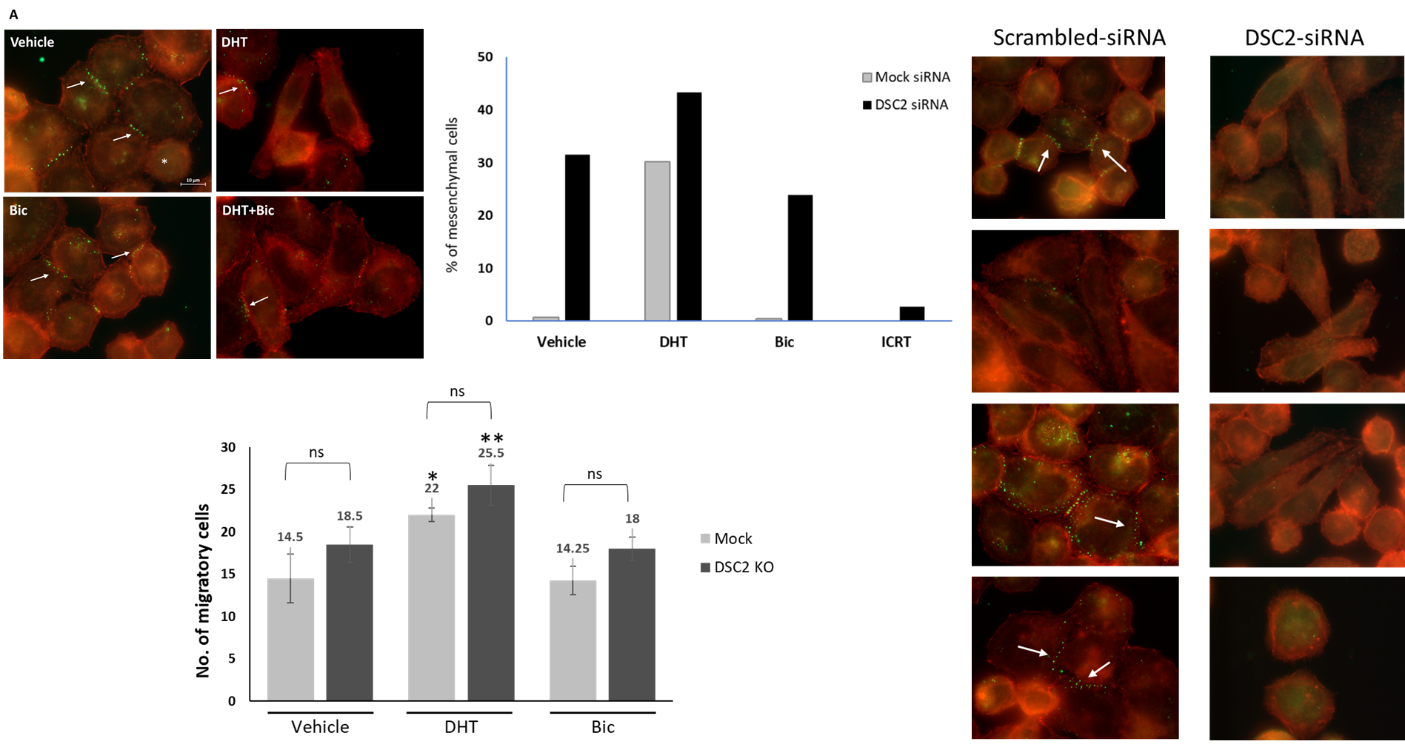
Desmosomes are cellular structures that are critical in cell-cell adhesion and in maintaining tissue architecture. Changes in the expression of desmocollin-2 (DSC2) have been noted during tumor progression into an invasive phenotype and as cells undergo epithelial-mesenchymal transition. We have previously reported that breast MDA-MB-453 cancer cells, a luminal androgen receptor (AR) model of triple-negative breast cancer, acquire mesenchymal features when treated with the AR agonist, dihydrotestosterone (DHT). We have therefore investigated androgen regulation of the expression and cellular localization of DSC2 in MDA-MB-453 cells. Treatment of the cells with DHT resulted in a dose-dependent reduction in DSC2 protein levels and dispersion of its membrane localization concomitant with AR- and β-catenin-mediated mesenchymal transition of cells. A significant correlation was revealed between decreased expression of AR and increased expression of DSC2 in patient samples. In addition, whereas lower expression of AR was associated with a reduced overall and recurrence-free survival of breast cancer patients, higher expression of DSC2 was found in invasive breast tumors than in normal breast cells and was correlated with lower patient survival. Upon knocking down DSC2, the cells became elongated, mesenchymal-like, and slightly, but insignificantly, more migratory. The addition of DHT further stimulated cell elongation and migration. DSC2 siRNA-transfected cells reverted to a normal epithelial morphology upon inhibition of β-catenin. These results highlight the role of DSC2 in maintaining the epithelial morphology of MDA-MB-453 cells and the negative regulation of the desmosomal protein by DHT during stimulation of the androgen-induced, β-catenin-mediated mesenchymal transition of the cells.

3- Dihydrotestosterone Induces Chemo-Resistance of Triple-Negative Breast MDA-MB-231 Cancer Cells Towards Doxorubicin Independent of ABCG2 and miR-328-3p.
Curr Mol Pharmacol. 2021;14(5):860-870. doi: 10.2174/1874467214666210531170355. Al-Momany B, Hammad H, Ahram M.
BACKGROUND:
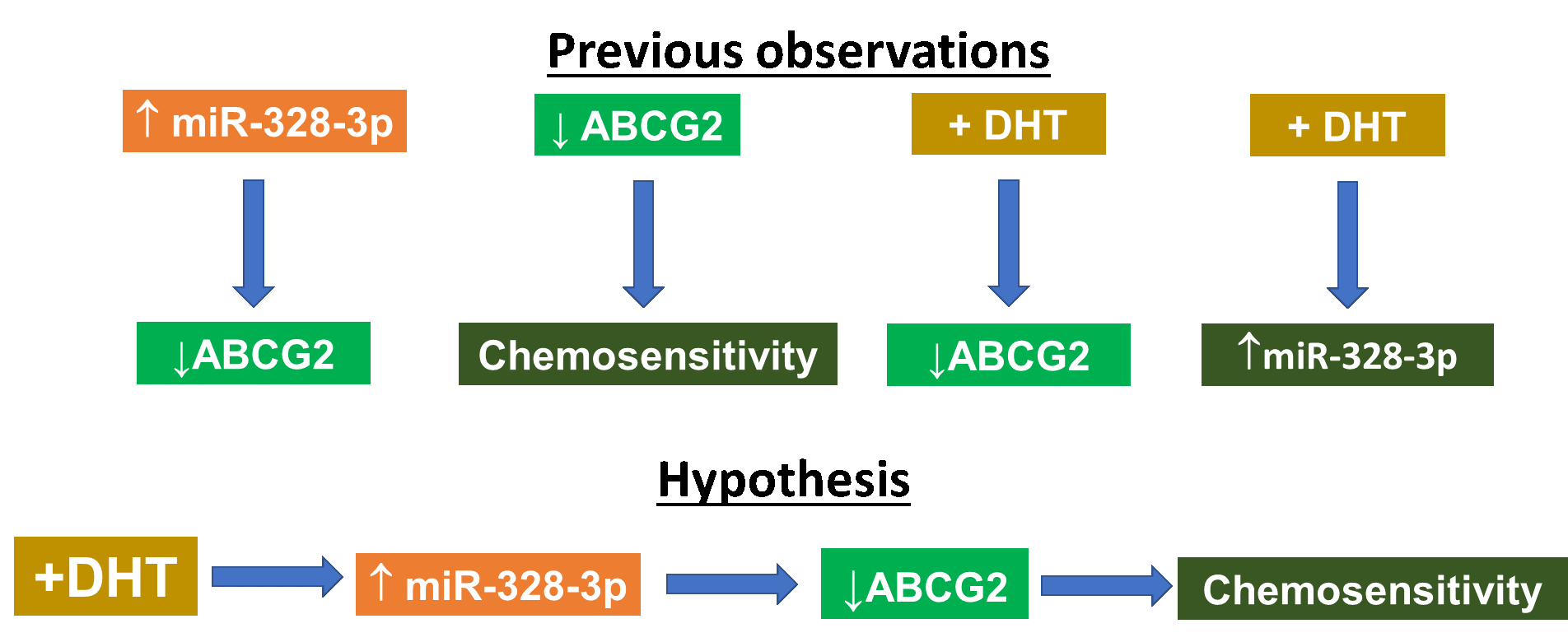
Androgens potentially have an important role in the biology of breast cancer, particularly triple-negative breast cancer (TNBC). Androgen receptor (AR) may offer a novel therapeutic strategy, including the use of microRNA (miRNA) molecules. We have previously shown that AR agonist, dihydrotestosterone (DHT), increases the expression of miR-328-3p in the TNBC MDA-MB-231 cells. One target of the latter miRNA is ATP-binding cassette subfamily G member 2 (ABCG2), which modulates the chemo-response of cancer cells by pumping out xenobiotics.
OBJECTIVE:
Using MDA-MB-231 cells as a model system for TNBC, we hypothesized that DHT would induce cell sensitivity towards doxorubicin via increasing levels of miR-328-3p and, consequently, reducing ABCG2 levels.
METHODS:
Chemo-response of cells towards doxorubicin, tamoxifen, and mitoxantrone was evaluated using cell viability MTT assay. Cells were transfected with both miR-328-3p mimic or antisense molecules. Real-time PCR was utilized to assess RNA levels and immunoblotting was performed to investigate levels of ABCG2 protein. PCR arrays were used to assess changes in the expression of drug response regulatory genes.
RESULTS:
Contrary to our hypothesis, treating MDA-MB-231 cells with DHT no effect towards tamoxifen or mitoxantrone, increased cell resistance towards doxorubicin was noted, concomitant with decreased expression of ABCG2. This under-expression of ABCG2 was also found in MCF-7 and MDA-MB-453 cells treated with DHT. Although miR-328-3p decreased ABCG2 mRNA and protein levels, the miRNA did not alter the chemo-response of cells towards doxorubicin and did not affect DHT-induced chemo-resistance. AR activation slightly decreased the expression of 5 genes, including insulin-like growth factor 1 receptor that may explain the mechanism of DHT-induced chemo-resistance of cells.
CONCLUSION:
DHT regulates chemo-response via a mechanism independent of ABCG2 and miR-328-3p.
4- Involvement of β-catenin in Androgen-induced Mesenchymal Transition of Breast MDA-MB-453 Cancer Cells.
Endocr Res. 2021 Aug;46(3):114-128. doi: 10.1080/07435800.2021.1895829. Epub 2021 Mar 11. Ahram M, Bawadi R, Abdullah MS, Alsafadi DB, Abaza H, Abdallah S, Mustafa E.
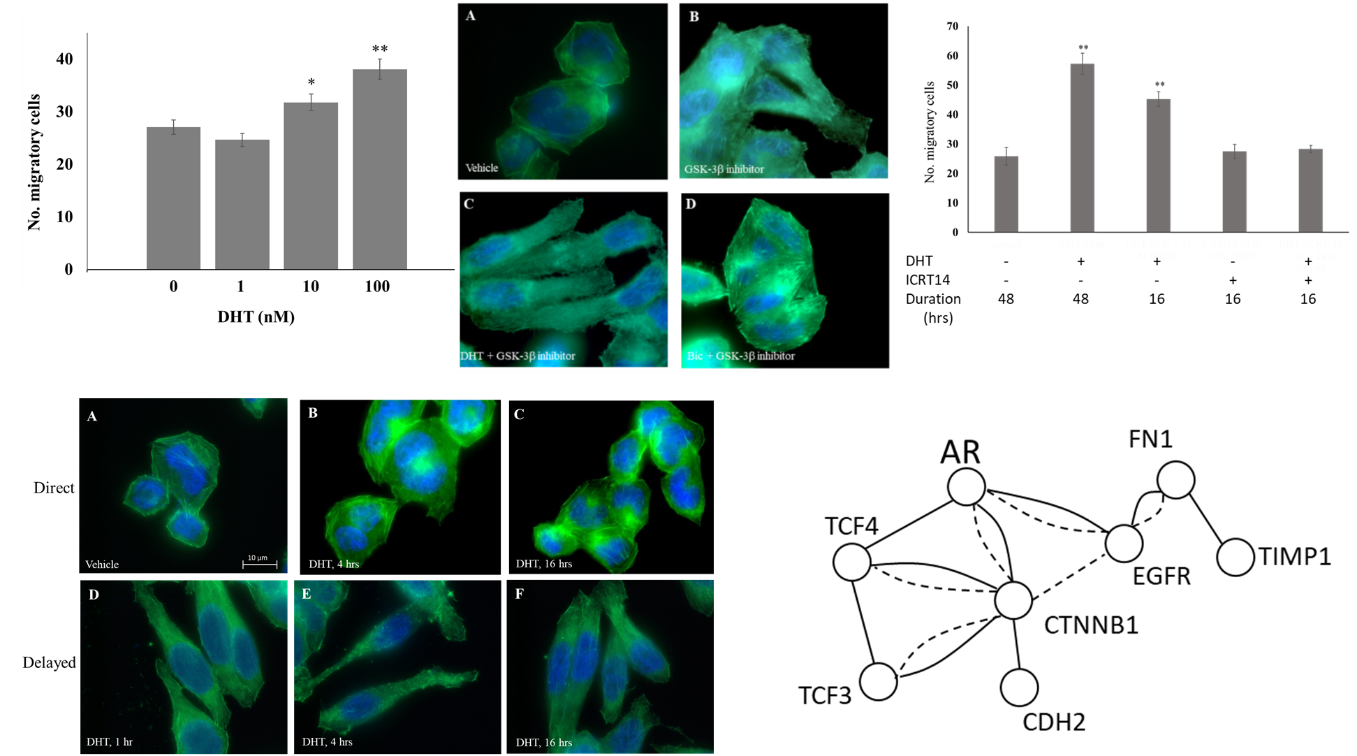
Purpose The cellular and molecular dynamics of DHT-induced EMT in MDA-MB-453 cells were investigated. Methods:PCR arrays were used to examine the expression of EMT-regulatory genes. Immunoblotting was used to detect protein levels and confirm protein-protein interaction following immunoprecipitation. Immunofluorescence was used to observe rearrangement of the actin cytoskeleton and cell morphology. Cell migration was assessed by transwell assay. Results: Change of cell morphology was concomitant with increased cell migration after treating cells with DHT. Exposure of cells to DHT for one hour was sufficient to induce changes in cell morphology and actin cytoskeleton after 72 hours indicating altered gene expression. A long-term lasting nuclear translocation of AR was observed after a short exposure of cells to DHT. Investigating the expression of 84 EMT-related genes revealed down-expression of β-catenin, N-cadherin, and TCF-4 and increased expression of Slug, all of which were confirmed at the protein level. Yet, not only early interaction of AR and β-catenin was observed following AR activation, inhibition of β-catenin blocked DHT-induced mesenchymal transition and migration. Wnt signaling was found to be partially important in DHT-induced morphological alteration. The mesenchymal transition of cells could be induced by treating cells with an inhibitor of glycogen synthase kinase-3β, an enzyme that inhibits β-catenin; this morphological transition could be reversed by antagonizing AR suggesting that AR functions downstream of β-catenin. Conclusions: These results suggest that MDA-MB-453 cells undergo partial EMT induced by DHT, β-catenin is critical for this phenotypic change, and AR probably reciprocally mediates the mesenchymal transition of these cells upon activation of GSK-3 β.

5- Dihydrotestosterone regulates expression of CD44 via miR-328-3p in triple-negative breast cancer cells.
Gene. 2018 Oct 30;675:128-135. doi: 10.1016/j.gene.2018.06.094. Epub 2018 Jun 28. Al-Othman N(1), Hammad H(1), Ahram M(2).
Triple-negative breast cancer (TNBC) is an aggressive subtype that lacks effective targeted therapeutics strategy and has poor prognosis. Targeting androgen receptor (AR) in TNBC is thought to be a promising approach. We hypothesized that AR, functioning as a transcription factor, controls cell behavior via regulating the expression of microRNA molecules (miRNAs). The expression of 84 breast cancer-specific miRNAs in MDA-MB-231 cells, a highly invasive TNBC model system, was investigated using PCR arrays following treatment of cells with 5α-dihydrotestosterone (DHT). The expression of 33 miRNAs was changed by more than 2 folds including miR-328-3p, which was up-regulated by 13 folds. Transfection of cells with either miR-328-3p mimic or anti-sense molecules decreased cell motility. DHT-mediated effect on the expression and function of CD44, a target of miR-328-3p, was investigated. CD44 expression and cell adhesion to hyaluronic acid (HA) were down-regulated when cells were treated with DHT or transfection with a miR-328-3p mimic. On the other hand, the AR antagonist, bicalutamide, or transfection of cells with miR-328-3p anti-sense molecules had the opposite effect. Cells transfected with miR-328-3p anti-sense molecules reduced the negative effect of DHT on CD44 expression and cell adhesion to HA. In addition, DHT further reduced the expression of CD44 and cell adhesion to HA in cells transfected with miR-328-3p mimic. These results strongly suggest that miRNAs can mediate AR regulation of breast cancer cells and that AR controls the expression of CD44 via miRNA-dependent and independent mechanisms.