Backgrounds
The concept of a “magic bullet" still inspires researchers to develop targeted drug delivery systems that save normal cells and reach diseased ones. The unmet cancer treatment needs fit this paradigm, highlighting the importance of developing targeted drug delivery systems. Most of nanomedicines lack specificity towards their target cells. This is why a promising approach in this field consists in designing carriers that are functionalized with ligands that support targeting and drug delivery in a selective manner. Several types of targeting ligands can be used to guide nanocarriers to tumor cells including small molecules, proteins, peptides, carbohydrates, antibodies, and aptamers.

Cancer is a major health problem affecting millions yearly with a very high mortality rate. Although many drugs have been explored and shown promising antitumor efficacy, most of these drugs belong to a class of molecules that challanged low aqueous solubility and poor cellular uptake, leading to a lack of therapeutic efficacy and unwanted side effects. Cancer nanomedicine was developed to overcome the limitations of conventional cancer therapies by applying different nanotechnologies that render cancer treatment safer and more effective. Those technologies encompass diagnostic applications, therapies, and cancer targeting. The advantages of delivering anticancer agents using nanoparticles include good pharmacokinetics, precise targeting of tumor cells, and minimizing or eliminating drug resistance and side effects.

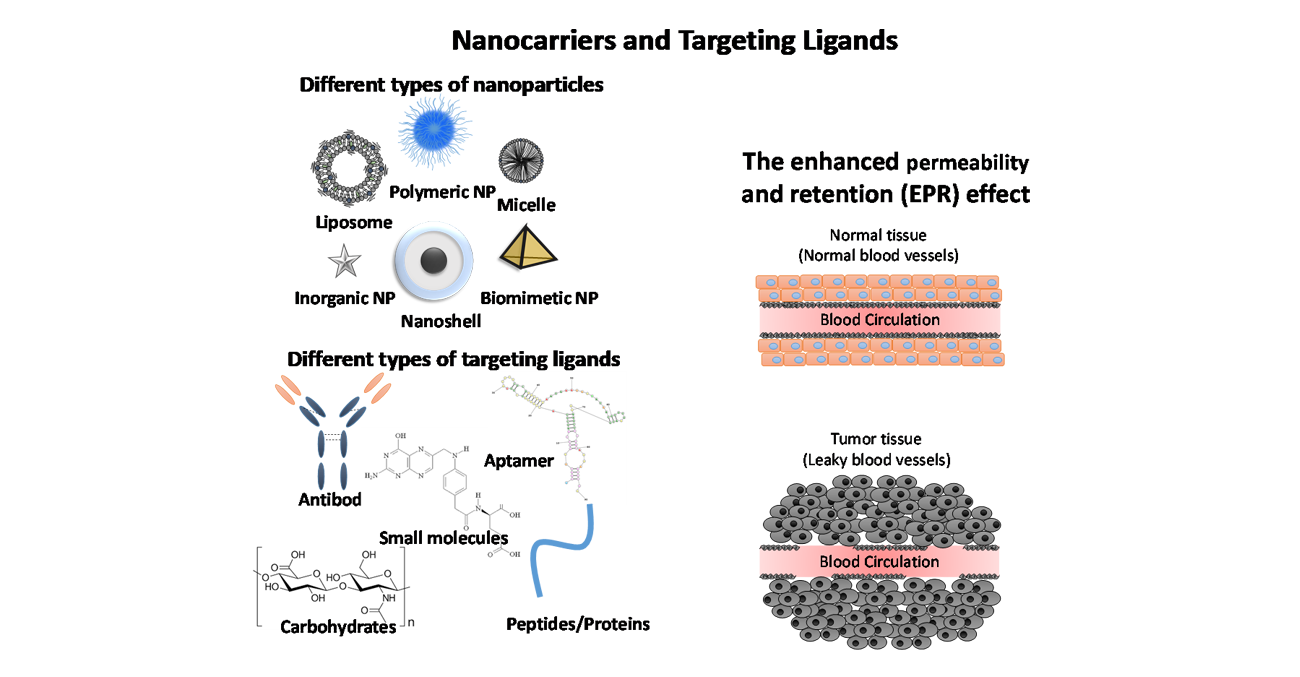
Aptamers are synthetic single-stranded RNA or DNA oligonucleotides that can be selected against almost any target, from small molecules to whole cells. Their unique structure enables high affinity binding to their targets, while offering significant advantages over antibodies, such as low immunogenicity, greater stability, and ease of chemical modification. Given their promising attributes, aptamers have attracted much attention as molecular ligands for cancer cell detection and targeted cancer therapy. Importantly, aptamers can be coupled to the surface of drug-loaded nanomedicines to enable selective uptake and more efficient cancer cell targeting. Nanotechnology has enabled targeted delivery of these drugs to cancer cells, which has led to a significant reduction in their systemic side effects. Several types of nanomedicines have been reported to date, with promising potential to treat various diseases, especially cancer.
Preliminary work achived and published by our group
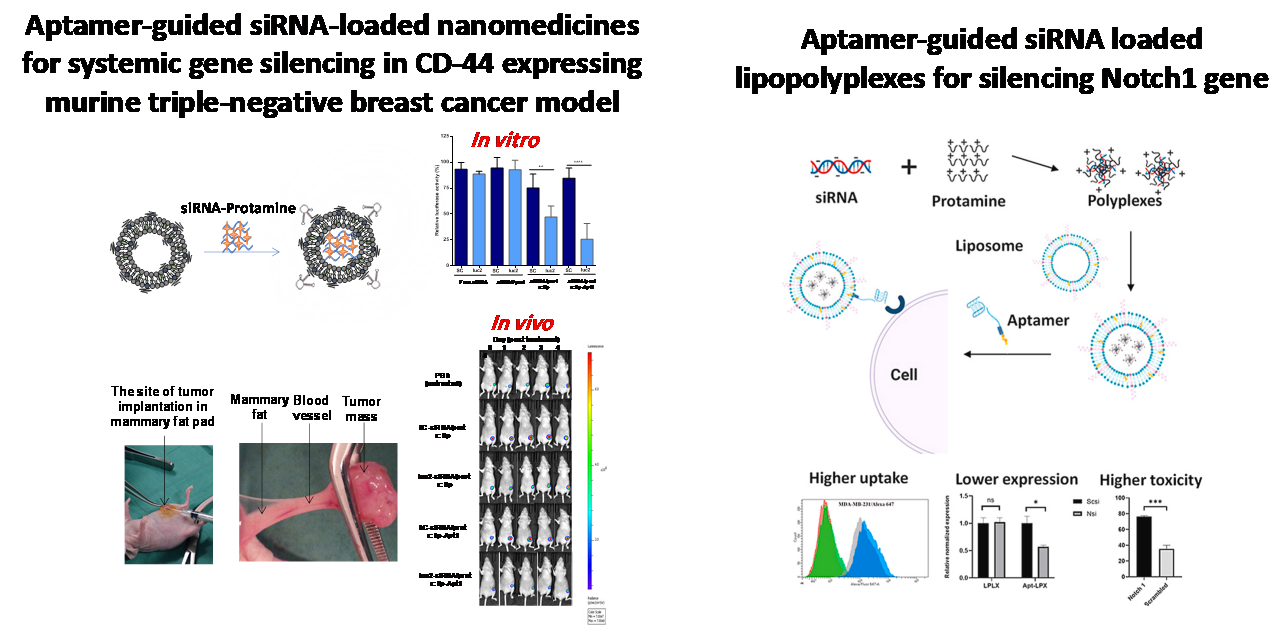
Aptamer-functionalized nanoparticles for siRNA delivery
In this work, we selected a modified RNA aptamer, Apt1, to bind the human CD44 receptor protein with high affinity using the SELEX method. The selected aptamer was modified with 2'-F-pyrimidine to increase stability against nucleases for biological applications. Furthermore, we developed an aptamer-functionalized-liposome loaded with siRNA molecules as a model of a drug delivery system that selectively targets CD44-expressing tumor cells
in vitro and
in vivo. Such functionalization was performed by thiol-maleimide conjugation chemistry between 3'-thiol-modified Apt1 and the maleimide functionalized to the surface of the liposomes. The targeted liposomes expressed high affinity for CD44-positive cells without triggering any inflammatory response within these cells. Moreover, the data of this study showed higher inhibition of targeted gene by the aptamer functionalized liposomes loaded with siRNA
in vitro and prolonged inhibition
in vivo. In a different work, an aptamer functionalized lipopolyplexes loaded with siRNA for silencing Notch 1 gene was developed. As a cationic protein, Protamine was used to condense siRNA and facilitate loading into PEGylated liposomes. The resulting siRNA-loaded lipopolyplexes were then functionalized with anti-nucleolin aptamer as a targeting ligand for selective delivery of Notch 1 siRNA into nucleolin-expressing triple-negative breast cancer cells. The results of this study showed a higher cellular uptake, Notch 1 silencing, and anti-proliferative effect for the aptamer functionalized lipopolyplexes compared to plain lipopolyplexes.
The current targeted siRNA delivery system can be used for other siRNA molecules for silencing different genes in different cells. More in vivo studies are important and can provide valuable information about the therapeutic potency of this siRNA delivery system, especially in types of cancers in which the therapeutic options are limited, such as triple-negative breast cancer.
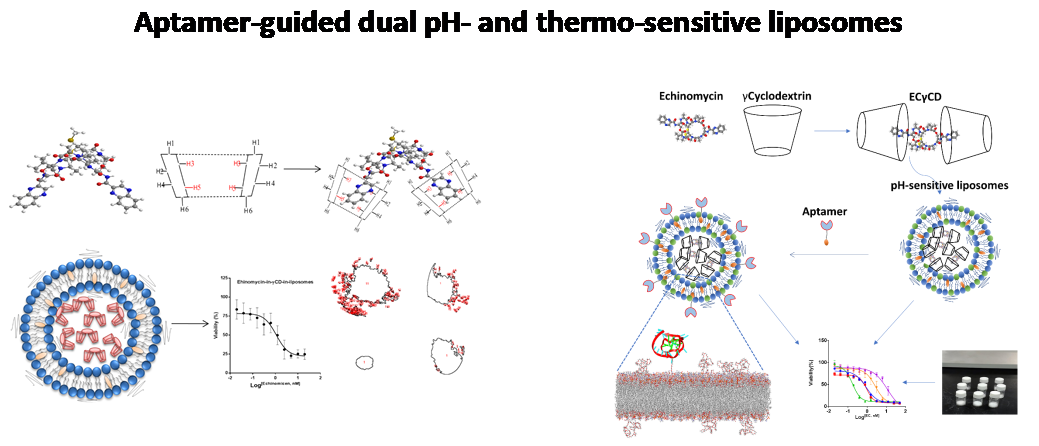
Aptamer-functionalized nanoparticles for hydrophobic drugs delivery
In this work, we show for the first time the complexation of echinomycin with γCD (echinomycin-in-γCD) and the loading into PEGylated liposomes (echinomycin-in-γCD-in-liposomes). The liposomes encapsulating echinomycin showed potent anti-proliferative and anti-invasive effects against U-87 MG glioblastoma cell line. In separate work, our group developed smart and multifunctional aptamer-guided and PEGylated pH-sensitive liposomes that were designed, formulated, and fully characterized. These liposomes were stable at physiological pH and released their payload at low pH. Particle size, size distribution, and charge stability were evaluated at storage and physiological temperatures. These smart-multifunctional liposomes were tested for pH sensitivity and release using calcein dye and echinomycin. The dye and the potent anticancer antibiotic, echinomycin, were successfully encapsulated inside liposomes. AptNCL-PEGLippH-EC-γCD exhibited excellent selectivity and cytotoxic activity on three cancer cell lines (MCF7, MDA-MB-231, and A549) compared to normal cells. The current aptamer-guided pH-sensitive liposomes can be used as a delivery system for other therapeutic molecules. Moreover, in vivo and mechanistic studies can provide valuable information about the antitumor efficacy and more understanding of the mechanism of selective uptake by cancer cells.

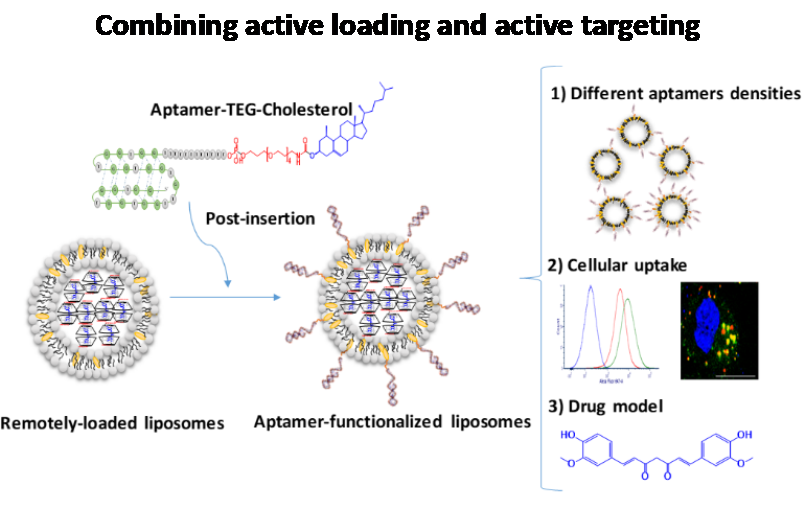
In a separate work, we combined active loading and active targeting DDS and developed a new aptamer-liposomes conjugation strategy based on a post-insertion approach. Cholesterol-attached NCL aptamer was successfully post-inserted in the bilayer of actively loaded preformed liposomes that were reported in our previous work. Cellular uptake studies, either by flow cytometry or CLSM, showed the key role of aptamer conjugation for liposomes active targeting and entry into the cancer cells. The NCL aptamer functionalized liposomes actively loaded with curcumin payload enhanced cellular internalization toxicity to MDA-MB-231 and MCF-7 breast cancer cells as compared to non-functionalized liposomes.
Main Contact:
Dr. Walhan Alshaer
Cell Therapy Center - The University of Jordan
Queen Rania Street, Amman, Jordan
Phone: +96265355000; ext: 23960
Mobile: +962790823678
Email: walhan.alshaer@ju.edu.jo
Research group and collaborators:
Prof. Elias Fattal
Université Paris-Saclay, CNRS, Institut Galien Paris-Saclay, 91400, Orsay, France
Prof.
Said I. Ismail
Qatar Genome Project, Qatar Foundation, Doha 5825, Qatar
Prof. Fadwa Odeh
Department of Chemistry, School of Science, The University of Jordan, Amman, 11942, Jordan
Prof.
Abeer Al Bawab
Department of Chemistry, School of Science, The University of Jordan, Amman, 11942, Jordan
Prof. Malek Zihlif
Department of Pharmacology, Faculty of Medicine, The University of Jordan, Amman, Jordan
Prof. Yasser Bustanji
Department of Clinical Pharmacy, Faculty of Pharmacy, The University of Jordan, Amman, Jordan
Dr. Manar Zraikat
Department of Pharmacology, Faculty of Medicine, The University of Jordan, Amman, Jordan
Dr. Hamdi Nsairat
Pharmacological and Diagnostic Research Center, Faculty of Pharmacy, Al–Ahliyya Amman University, Amman, 19328, Jordan
Dr. Zainab Lafi
Pharmacological and Diagnostic Research Center, Faculty of Pharmacy, Al–Ahliyya Amman University, Amman, 19328, Jordan.
Dr. Lobna Gharaibeh
Pharmacological and Diagnostic Research Center, Faculty of Pharmacy, Al–Ahliyya Amman University, Amman, 19328, Jordan
Selected publications:
[1] Z. Lafi, W. Alshaer, M.m.M. Hatmal, M. Zihlif, D.A. Alqudah, H. Nsairat, H. Azzam, T. Aburjai, Y. Bustanji, A. Awidi, Aptamer-functionalized pH-sensitive liposomes for a selective delivery of echinomycin into cancer cells, RSC Advances, 11 (2021), pp.29164-29177, 10.1039/D1RA05138E.
[2] L. Gharaibeh, W. Alshaer, S. Wehaibi, R. Al Buqain, D.A. Alqudah, A. Al-Kadash, H. Al-Azzawi, A. Awidi, Y. Bustanji, Fabrication of aptamer-guided siRNA loaded lipopolyplexes for gene silencing of notch 1 in MDA-mb-231 triple negative breast cancer cell line, Journal of Drug Delivery Science and Technology, 65 (2021), pp.102733.
[3] H. Nsairat, I.S. Mahmoud, F. Odeh, D. Abuarqoub, H. Al-Azzawi, R. Zaza, M.I. Qadri, S. Ismail, A. Al Bawab, A. Awidi, W. Alshaer, Grafting of anti-nucleolin aptamer into preformed and remotely loaded liposomes through aptamer-cholesterol post-insertion, RSC Advances, 10 (2020), pp.36219-36229, 10.1039/D0RA07325C.
[4] F. Odeh, H. Nsairat, W. Alshaer, M.A. Ismail, E. Esawi, B. Qaqish, A.A. Bawab, S.I. Ismail, Aptamers Chemistry: Chemical Modifications and Conjugation Strategies, Molecules, 25 (2019), 10.3390/molecules25010003.
[5] W. Alshaer, H. Hillaireau, E. Fattal, Aptamer-guided nanomedicines for anticancer drug delivery, Adv Drug Deliv Rev, 134 (2018), pp.122-137, 10.1016/j.addr.2018.09.011.
[6] S.I. Ismail, W. Alshaer, Therapeutic aptamers in discovery, preclinical and clinical stages, Adv Drug Deliv Rev, 134 (2018), pp.51-64, 10.1016/j.addr.2018.08.006.
[7] W. Alshaer, H. Hillaireau, J. Vergnaud, S. Mura, C. Delomenie, F. Sauvage, S. Ismail, E. Fattal, Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model, J Control Release, 271 (2018), pp.98-106, 10.1016/j.jconrel.2017.12.022.
[8] W. Alshaer, H. Hillaireau, J. Vergnaud, S. Ismail, E. Fattal, Functionalizing liposomes with anti-CD44 aptamer for selective targeting of cancer cells, Bioconjugate chemistry, 26 (2015), pp.1307-1313.