Corneal wounds and abrasions are relatively common, they comprise 8 percent of all eye presentations in primary care and are among the most common eye conditions seen in emergency departments, furthermore, an intentional removal of the eye's clear protective outer layer of the corneal surface is the first stage in refractive (laser) eye surgeries. It is estimated that 30 million people undergo laser eye surgery yearly.
The treatment goals in cases of corneal abrasion are the relief of pain, the prevention of bacterial infection, and speeding the healing process. Treatment options include oral analgesics, as well as topical agents, such as antibiotics and anti-inflammatory drugs.
Few treatments have been evaluated in controlled trials, and many recommendations are based on theoretical benefit and consensus. Complications of a corneal abrasion can be severe and may lead to blindness if not treated correctly.
One of the most promising treatments for corneal abrasion is vitamin C, a potent antioxidant. Vitamin C can accelerate corneal wound healing after high oral doses as effervescent tablets or intravenous administration. However, while intravenous administration is deemed inconvenient for patients with corneal abrasion, high oral doses can also be associated with side effects that vary from stomach cramps or bloating to heartburn and skin flushing and can result in the formation of kidney stones.
Instead, the UJ team of researchers attempted to develop a topical eye drop formulation expected to result in high concentrations of vitamin C in the cornea while avoiding systemic side effects associated with the use of a high dose of effervescent tablets.
“The concept was simple, topical eye drop application is more patient-friendly and was expected to have better results", said
Hatim AlKhatib, Professor of Pharmaceutical Technology at the University of Jordan and the principal author of a paper describing the innovative eye drop formulation.
AlKhatib continued: “But we know that vitamin C administration in a conventional eye drop will present two big obstacles against the proof of our concept: vitamin C is chemically unstable oxidizing rapidly in solutions and its permeability through the cellular membranes and biological tissues is poor".
So, a team of graduate students at the School of Pharmacy Mai Jaber and Tasnim Riyal, ophthalmologist Bahaa Aldin Jaber, and Dr. Walhan Alshaer of the Cell Therapy Center started working on the problem with Prof. AlKhatib in collaboration with Prof. Saja Hamed of the Hashemite University in Zarqa.
The formulation they developed was a solid in oil nanodispersion of vitamin C that can solve the challenges of vitamin C delivery in a topical preparation, explains Prof. AlKhatib: “Vitamin C in the formulation is present as small nanoparticles of about 40 nm in diameter with a surface covered with the hydrophobic part of a surface-active agent. This improves both the chemical stability of vitamin C and its permeability through cellular membranes and biological tissues".

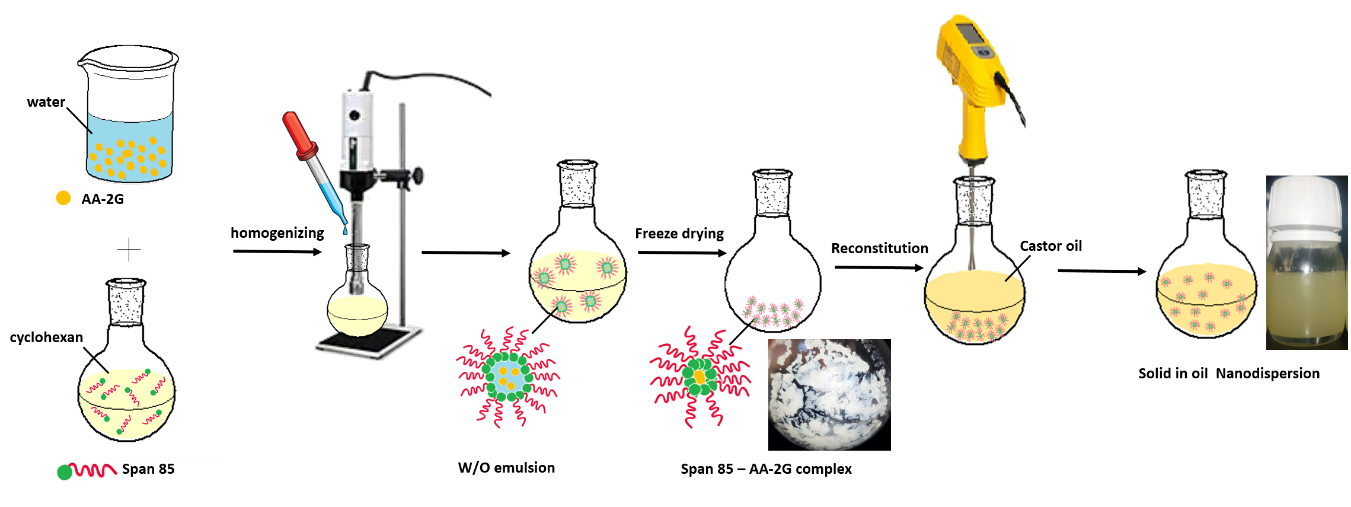
Schematic presentation of the preparation of Vitamin C nanodispersions.
The formulation was shown to result in improved permeation through the cornea in the lab and accelerated corneal wound healing in laboratory animals.

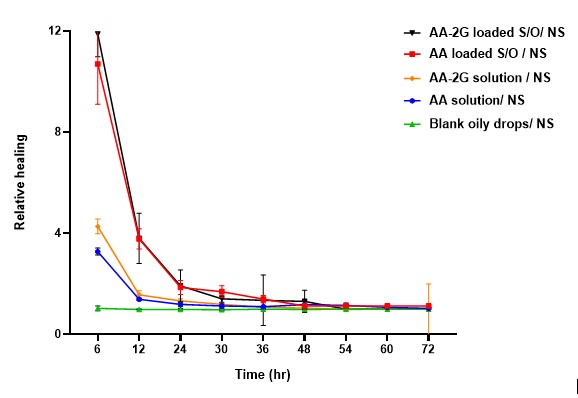
Relative healing of Vitamin C solution, Vitamin C Glucoside solution, Vitamin C S/O nanodispersion eye drops or Vitamin C Glucoside S/O nanodispersion oily eye drops and blank oily eye drops (to normal saline) over
a 72-hour period post corneal debridement.
The researchers were also able to photograph the particles' penetration into the corneal tissue and uptake by cells using confocal and florescence microscopy.

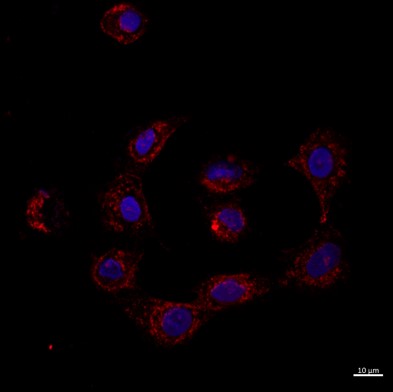
Confocal image of A549 cell line for cellular uptake evaluation of vitamin C from S/O/W nano emulsion
after 24 hours of incubation, 63X with the nanoparticles labeled with Rhodamine B showing in red around a DAPI stained cell nuclei in blue.
The results of the project were granted a
PCT patent and published in the
International Journal of Pharmaceutics.
Prof. AlKhatib describes the results as: “an example of the innovation culture at the UJ and the strength of the interdepartmental collaboration translating into solutions that meet patient needs".
He elaborated: “we are now looking to collaborate with investors or pharmaceutical manufacturers to fund the development of a scaled-up manufacturing process and the clinical testing of the formulation in humans to improve the quality of life of corneal abrasion patients by introducing new standard treatments for their condition".

Resources:
Link to the UJ PCT patent application describing the innovative formulation.
Paper published by researchers on the topic.
Website and contact information of Prof. Hatim AlKhatib